If the formation of molecules in
modern chemistry one way or another explained, the reasons of formation of
solid bodies, on a view of the author, are unconvincing, as at a level of
molecules all connections are already saturated and, behind elimination of
"hydrogen bond" and the electrostatic bond is not present sufficient
energy opportunities to form of molecules solid macrobody.
|
|
|
|
|
|
From a point of view of the above-stated types of a chemical bond, in actual
objects, the connection, basically, never can be completely saturated because
of the steric factors. Therefore always there is an opportunity not only to
collect from molecules macrobody, but also for it macrobody the ability to
affixing of foreign atoms is maintained, that is exhibited in its ability to a
sorption, dissolution, chemical activity etc.
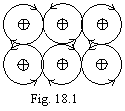
Let's analyses some examples. On a figure 18.1 the variety of a
one-electron bond in metals is figured.
The trajectory of a
casual electron is shown arrows. From a figure it is visible, why the metals
enable major plastic strains. The similar connection is carried out in metal
simultaneously many electrons in the most miscellaneous directions.
In overwhelming
majority of non-metallic matters the connection of component particles has
certain directedness, therefore they friable. It is possible to formulate a
blanket apparent rule: are more saturated the connection in a molecule - the
more feeble connection between molecules. For example, structure NH3
(fig.18.2.a). In it only one connection is not saturated and in standard
conditions NH3 - gas.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In H2O (fig. 18.2.b) two connections are not saturated and in
standard conditions H2O - fluid. In NaCl (fig.
18.2.c) not saturated already three connections, therefore NaCl - solid
matter. For BeO (fig. 18.2.d) besides that three connections are not
saturated also the beryllium donates one electron to oxygen completely, i.e. in
solid oxide BeO except for saturation of one-electron bonds the strong
electrostatic bond acts also. Therefore BeO will forms a very strong
crystal lattice in matching with NaCl.
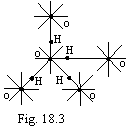
As an example, on a figure 18.3 the
crystal lattice of ice as a tetrahedron is figured. Each atom of oxygen is
enclosed by four atoms of hydrogen two of which one are arranged close. All
one-electron bonds for oxygen and hydrogen in this case are saturated.
|
|
|
|
|
|
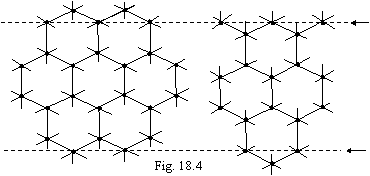
Following of added up tradition, we shall consider in conclusion of this
section of structure of diamond and graphite. As the atom of carbon has in a
torus four electrons that, joining each atom with four neighbors in tetrahedral
structure with completely saturated connections (it will be a two-electronic
bond), we shall receive structure of diamond, where each atom of carbon has 8
electrons. If is carried out not two-electronic, and one-electron bond between
atoms of carbon, the flat hexagonal lattice will be formed, in which one half
of atoms of carbon has 5 electrons, and other half - 6, as shown in a figure
18.4.
The structure of graphite is gained
by superimposition of planes against each other (along dashed lines). Thus the
atoms of carbon having till 6 electrons, will not form connection, and the
atoms having till 5 electrons appear against each other and will forms an
one-electron bond. Thus, in a lattice of graphite all atoms of carbon have till
6 electrons, accordingly, the strength it is much lower, than for diamond.
Here are
circumscribed, behind a deficiency of a place, only principled fundamentals of
new views on a chemical bond and are given examples of a small number of
compounds. The reader can check the author on a word, that he has analyzed
major number of different chemical compounds and has not found anything that
contradicts above-stated, though the chemistry has business with huge variety
of compounds.
18.1. About "surface gas" of liquids
and solids
Concept about
surface gas of liquids and solids.
It helps to explain
their many properties and to give the quantitative theory of these properties.
Atoms or molecules, of which the solids or liquid consists, are capable to jump
out of volume on a surface and freely enough to move on it, jumping from one
potential hole in another, as a power barrier to such jumps small. If energy of
activation of transition in surface gas large enough, the concentration of
molecules in surface gas, accordingly, is small, and it is possible to consider
it as ideal bivariate gas.
Concentration of
particles in surface gas.
Speed of transition
of particles in surface gas:
![]() (18.1.1.),
(18.1.1.),
where: C0
- surface concentration of particles of substance, 1/cm2
C - concentration of particles in surface gas.
Speed of condensation
from surface gas in volume of substance:
![]() (18.1.2.).
(18.1.2.).
The balance is
established quickly, since the process of condensation has no energy of
activation:
![]() (18.1.3.).
(18.1.3.).
(18.1.1.) and
(18.1.2.) we shall substitute in (18.1.3.) and we shall find:
![]() (18.1.4.).
(18.1.4.).
On the Arrhenius:
![]() ,
, ![]() , since E2=0
(18.1.5.),
, since E2=0
(18.1.5.),
where: E1
and E2 - energy of activation, accordingly, transition of a
particle in surface gas and condensation in volume of substance, erg/mol.
(18.1.5.) we shall
substitute in (18.1.4.) and at ![]() (particle same), we shall find:
(particle same), we shall find:
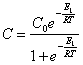 (18.1.6.).
(18.1.6.).
At E1>>RT
(18.1.6.) will accept a kind:
![]() (18.1.7.).
(18.1.7.).
Than closer to a
condition fusing of a solid or boiling of a liquid, the below E1
and at E1=0 (18.1.6) will accept a kind: C=C0/2
that is obvious.
The energy of
activation of carry of a particle in surface gas will be equal to the sum:
![]() (18.1.8.),
(18.1.8.),
where: Q -
energy necessary for break of connections with atoms of volume of substance at
given temperature, cal/mol,
F - specific free surface energy.
QT we shall find from the following
reasons. To break off connections in a liquid at given temperature, it needs to
be heated up to temperature of boiling and to evaporate, then to cool of vapour
up to initial temperature, but without condensation. To break off connections
in a solid, it is necessary it to heat up to Tmel and melted.
In the formed liquid of connections are so weakened, that they can be neglected
as a first approximation and molecule or atoms to consider practically free.
The formed liquid should be cooled up to initial temperature without
crystallization in a solid. Thus it is necessary to notice, that the free
specific surface energy is automatically taken into account also. Therefore as
a first approximation (without cooling gas or liquid from temperature of phase
transition up to initial temperature). For concentration of particles in
surface gas such simplification is allowable:
![]() cal/mol
(18.1.9.),
cal/mol
(18.1.9.),
where: cp
- thermal capacity, cal/g×deg,
M - molecular weight, g,
![]() T=Tph-T,
where Tph - temperature of phase transformation, deg,
T=Tph-T,
where Tph - temperature of phase transformation, deg,
![]() 0 - specific heat melting or
evaporation, cal/mol.
0 - specific heat melting or
evaporation, cal/mol.
Formally, we can
attribute energy on (18.1.9.) to one atom. Thus we shall take into account,
that at an output in surface gas the atom has one degree of freedom, third of
this energy therefore suffices, it and will be energy of activation:
![]() (18.1.10.).
(18.1.10.).
Having substituted
(18.1.10.) in (18.1.7.), we shall find:
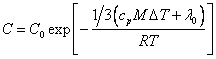 (18.1.11.).
(18.1.11.).
Expression (18.1.11.)
fairly far from temperature of boiling or melting, near to them it is necessary
(18.1.10.) to substitute in (18.1.6.):
 (18.1.12.).
(18.1.12.).
It is easy to show,
that:
![]() (18.1.13.),
(18.1.13.),
where: C0
- surface concentration of particles of substance, cm-2,
d - density of substance, g/cm3,
N0 – Avogadro constant, mol-1,
M - molecular
weight, g.
Surface tension.
Energy on the
equation (18.1.10.), come on one particle:
![]() (18.1.14.).
(18.1.14.).
Having multiplied
number of particles on (18.1.13.) on energy come on one particle on (18.1.14.),
we shall receive:
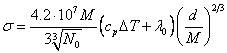 erg/cm2 (18.1.15.).
erg/cm2 (18.1.15.).
Expression (18.1.15.)
is possible to treat, how surface tension without account of surface gas, which
does not bring in contribution in ![]() .
.
In the table 18.1.1.
the calculated and experimental values of a surface tension for water and
mercury are submitted at 200С in erg/cm2.
Table 18.1.1.
|
Substance |
Is calculated on (18.1.15.)
|
Tabulated value
|
|
Water |
70 |
72.75 |
|
Mercury |
444 |
472 |
For flying liquids to
neglect concentration of atoms in surface gas it is impossible, since at change
of a surface these atoms do not render influence, therefore results of account
on (18.1.15.) it appear overestimated. For example, for ethyl alcohol the
calculated value on (18.1.15.) 56.7 erg/cm2, and experimental 22.8
erg/cm2. It is obvious, that the effective concentration of
particles on a surface influencing a surface tension, will be:
![]() (18.1.16.),
(18.1.16.),
where C is
determined by expression (18.1.12). If it to substitute in (18.1.16.) and to
multiply by energy comes on one particle, we shall receive:
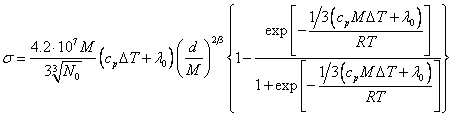 (18.1.17.).
(18.1.17.).
The known empirical
expression, suitable for many liquids, looks so:
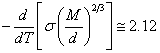 (18.1.18.).
(18.1.18.).
If to differentiate
on temperature (18.1.15.), as more simple, in comparison with adjusted
(18.1.17.), we shall receive the same result:
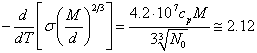 (18.1.19.).
(18.1.19.).
Evaporation heat.
Energy necessary for
heating and evaporation for a liquid at temperature of boiling we shall find on
a known ratio:
![]() (18.1.20.),
(18.1.20.),
where: ![]() - average thermal capacity of
a liquid, cal/mol×deg,
- average thermal capacity of
a liquid, cal/mol×deg,
![]() Tliq
= Tboil – T,
Tliq
= Tboil – T,
![]() tv – evaporation heat at temperature
of boiling, cal/mol.
tv – evaporation heat at temperature
of boiling, cal/mol.
Similarly for a
solid:
![]() (18.1.21.),
(18.1.21.),
где: ![]() - average thermal capacity of
a solid, cal/mol×deg,
- average thermal capacity of
a solid, cal/mol×deg,
![]() T1
= Tmelt – T,
T1
= Tmelt – T,
![]() melt – melting heat, cal/mol,
melt – melting heat, cal/mol,
![]() T2 = Tboil – Tmelt.
T2 = Tboil – Tmelt.
The formed gas as a
result of processes (18.1.20.) and (18.1.21.) has superfluous energy:
![]() (18.1.22.),
(18.1.22.),
where: ![]() - average thermal capacity of
gas, cal/mol,
- average thermal capacity of
gas, cal/mol,
![]() T = Tboil – T.
T = Tboil – T.
If we shall take into
account this circumstance, in result we shall receive for liquids:
![]() (18.1.23.)
(18.1.23.)
and for solids:
![]() (18.1.24.).
(18.1.24.).
As thermal capacity of
bodies depends on temperature, we have received approximate value evaporation
heat. To have exact value, it is necessary to know experimental value of the
expended integrated heat.
Comparison calculated
on (18.1.23.) and (18.1.24.) and experimental values of evaporation heat of
different substances at different temperatures is submitted in the table
18.1.2.
Table 18.1.2.
|
Substance |
Temperature, 0С |
|
|
|
CdCl2 |
568 |
41.2 |
41.1 |
|
Cs |
28.7 |
18.82 |
19.9 |
|
FeCl3 |
304 |
16.5 |
16.6 |
|
H2O |
0 |
10.77 |
10.80 |
|
H2O |
25 |
10.51 |
10.53 |
|
H2Se |
-65.7 |
5.34 |
5.61 |
|
Hg |
-38.9 |
15.20 |
15.73 |
|
Hg |
25 |
14.54 |
15.43 |
|
KBr |
735 |
48.9 |
51.1 |
Saturated vapor pressure.
It is obvious; that
values determined on the equations (18.1.23.) and (18.1.24.) will be energies
of activation of transition of particles from surface gas in a gas phase,
accordingly for a liquid and solid. Therefore we shall arrange one more check
of validity of the equation (18.1.23.) for further use. We use tabular data for
water (evaporation heat at 1000С 9717 cal/mol, ![]() =9.971 cal/mol×deg, 1 cal = 4.1858 joules). Results of
comparison of account on (18.1.23.) with the tabular data at different
temperatures are submitted in the table 18.1.3.
=9.971 cal/mol×deg, 1 cal = 4.1858 joules). Results of
comparison of account on (18.1.23.) with the tabular data at different
temperatures are submitted in the table 18.1.3.
Table 18.1.3.
|
Temperature, 0С |
Experimental
evaporation heat, joule/g |
The calculated
evaporation heat, joule/g |
|
0 |
2501 |
2492 |
|
5 |
2489 |
2480 |
|
10 |
2477 |
2469 |
|
15 |
2465 |
2457 |
|
20 |
2454 |
2446 |
|
25 |
2442 |
2434 |
|
30 |
2430 |
2423 |
|
40 |
2406 |
2399 |
|
50 |
2382 |
2376 |
|
100 |
2257 |
2260 |
The data of table
18.1.3. show that the equation (18.1.23.) is acceptable to rough accounts.
Let's consider that
the surface gas is ideal bivariate gas and submits to the known equation of a
gas condition:
![]() (18.1.25.),
(18.1.25.),
Number moles in
surface gas:
![]() (18.1.26).
(18.1.26).
In (18.1.25.) let's
substitute (18.1.26.) and value gas constant R:
![]() (18.1.27.),
(18.1.27.),
where the pressure is
expressed in atmospheres. If in (18.1.27.) let's substitute (18.1.12.) and
(18.1.13) with the account (18.1.23)., we shall receive finally:
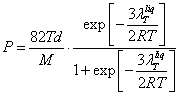 (18.1.28.).
(18.1.28.).
For solids it is
necessary to take into account not (18.1.23.), and (18.1.24.).