17. ELECTRON MOTION in a FIELD of TWO NUCLEI And CHEMICAL BOND
The viewing of this problem is the key moment in the theory of a chemical bond. I convert attention of the reader that the material enunciated in this chapter, completely is property of new physics (chemistry). The official science in the theory of a chemical bond prolongs to be carried with electronic "clouds", however, terming them already by "orbitals". The interaction of atoms is explained by "overlapping" of wave functions. In this connection to compare alternate chemistry there is nothing, except for an ionic bond, in which one the modern chemistry will utilizes classic notions, therefore has not differences with new physics.
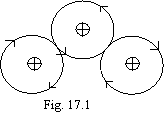 |
Apparently, that the electron motion in stationary atoms and molecules should happen is synchronize and so that the picture of an arrangement of electrons in a given instant periodically was iterated. Otherwise electrons will hinder each other in particular instants, when they, on necessity, should be in immediate proximity from each other, that on energy reasons it is impossible. Apparently as well that, as the electron is a rotative top (about it below), its motion is possible only in one plane. If in this plane there are some nuclei, the trajectory of a motion of an electron will be such, as is figured on a figure 17.1, that is a corollary of an sluggishness of an electron (with other things being equal it prefers to move rectilinearly).
Besides the electrons aim to be accumulated in a torus in possible a lot (see of fig. 15.7) up to 8 pieces except for the third and sixth electron to ensure a minimum of a potential energy at the expense of magnetic interaction of orbits. Except for hydrogen and helium, all remaining atoms as outside electrons have electrons forms a torus. The electrons of hydrogen and helium can be viewed in any quality.
As the magnetic interaction of electronic orbits plays the relevant role both at formation of atom, and at formation of molecules, it is necessary to specify, in what part of an ellipsoidal orbit the magnetic interaction is strongest. Apparently, that it is watched near to a nucleus because of a high speed of a motion of an electron and small radius of this motion. Therefore, without major injury for magnetic interaction, the orbit of an electron can be turned on a angle ±900 in orbital plane to ensure the greatest possible nuclear separation of atoms, thus, by ensuring a minimum of a potential energy. Therefore molecules from three atoms aim to form linear structure, of four - tetrahedron etc., forming a series of exact spatial figures, the contortions which one depend on a degree heteronuclear of components atoms, to be exact, from a degree of asymmetry of an electrostatic field. It is as a matter of convenience, we shall figure flat molecules in most cases.
The enunciated principles of a motion of an electron allow concretizing different types of a chemical bond.
This connection is characteristic for heteronuclear atoms with major asymmetry of a blanket electrostatic field, at which one the requirement of a synchronous motion of two electrons participating in connection is impossible to satisfy therefore connection is materializes one electron, as shown in fig. 17.1.1 (Z2>Z1).
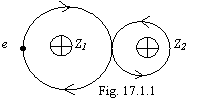 |
As it is visible from a figure, a majority of time the electron will spend between nuclei therefore formed molecule is invariable.
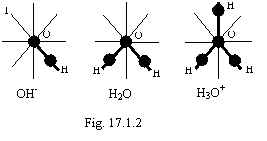 |
By example of chemical combinations with connection relevant of fig. 17.1.1 are compositions oxygen and hydrogen on a figure 17.1.2.
Orbits of electrons by way of dash us visuals from above, and they will forms a 8-electronic torus around of a nucleus of oxygen. Other electrons are not shown. The electronic orbits formed by an electron of hydrogen are marked out by thick strokes (in further it to do we shall not be because of an indistinguishability of electrons). To realize synchronization with an electron of connection, for an electron indicated in numeral 1 (OH-) is two opportunities, the choice from which one is determined by energy reasons. Or it is forced azimuthally to turn an orbit in a plane which is not conterminous to orbital plane of an electron, execute tie, or, in limits solved a blanket scoring of energy to deform an orbit so that synchronization has become possible. The second way is represented more possible, since the indispensable strain of an orbit is insignificant. As the formation of an 8-electronic torus is very profitably, the composition OH is forced to gain a missing electron from medium, forming hydroxyl and H3O is forced to donate an electron in medium, forming hydroxonium. Thus, the water represents an intermixture of particles figured on a figure 17.1.2. The adequacy of a figure to 17.1.2 actual facts is confirmed by that in solid hydrates of acids discover hydroxonium ion and relevant anion of an acid, instead of other particles. F. Cotton, G. Wilkinson "Modern inorganic chemistry", "World", М., 1969, 2 parts, page 14, tab. 6.1.
The principle of formation of molecules СН4 and Ccl4 is exact such, as is enunciated above, and the official chemistry is forced for this case to invent it’s the explanations. "In a ground state the atom of carbon has a configuration 1s22s22px2py and, therefore, has only two unpaired electrons. Therefore, it seems, it be necessary to expect, that with atoms X, having one unpaired electron (H, F, Cl etc.), the most inconvertible derivatives it should be of a type CX2. Certainly, it contradicts the facts - its more stable compositions with such atoms have structure of a type CX4, for example, CH4 and CCl4. To explain it, it is necessary to suspect, that the electronic configuration of atom of carbon varies so, that there are appears four unpaired electrons, how it is joined to four atoms X". Ibidem, 1 part, page 86.
As visuals, for a one-electron bond the concept of valence does not approach. As it will be visible from further, it is possible to tell and about other types bonds.
17.2. Connection with transmission of an electron
 |
 |
||
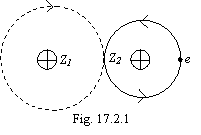
It is an extreme case of a one-electron bond, when the electron is completely transmitted in a shell of other atom that is figured on fig. 17.2.1.
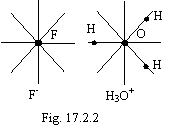
The example can be served by a solid hydrate HF×H2O, figured on a figure 17.2.2. The similar compounds will formed an ionic crystal lattice.
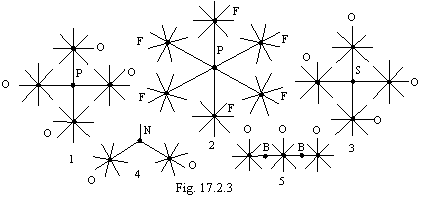 |
On a figure 17.2.3 some examples of combined connection one-electron and with transmission of an electron are given. 1 -
17.3. Two-electronic connection
 |
In spite of the fact that an one-electron bond, connection with transmission of an electron and two-electronic bond have the essential differences from the theory of the Lewis ("The basic idea of the theory of the Lewis is encompass, that the chemical bond is stipulated by that two atoms in common own one (or more) pair of electrons". F. Cotton, G. Wilkinson, Modern inorganic chemistry, World, М., 1969, 1 part, page 75), nevertheless, they are identical in main: at formation of a molecule the electrons are reallocated so that to form 8-electronic shell.
 |
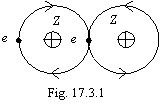
This connection is characteristic for homonuclear atoms, for atoms with identical structure of an outer shell, i.e. for cases of a symmetric blanket electrostatic field. This bonding is figured on a figure 17.3.1.
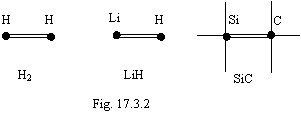
Three and more electrons already to be unable synchronously move on a trajectory therefore more than two electrons can not participate in connection of atoms. Connection is implemented at the expense of combined electrostatic and magnetic interaction. On a figure 17.3.2 the examples of such connection are given. It is indicated by double dash.
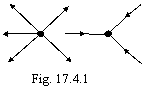 |
This type bond is not accompanied by socialization or transmission of electrons and is stipulated by building in of one toroidal electronic shell in another, as shown in a figure 17.4.1 (the current of traffic of electrons is shown arrows).
Such type bond, in opinion of the author, causes origin of unstoichiometric compounds.
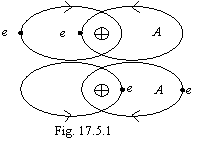 |
This connection concerns to a case of homonuclear atoms with only magnetic interaction of toroidal electronic shells (similarly to gravidynamic interaction of nucleons in nuclei, see theory of a nucleus), that reflexes on a figure 17.5.1. Apparently, that by a chemical compound with such bond the molecule A2 will be most typical, since the magnetic flux for limits of such molecule practically does not leave. Certainly, the formation of the extremely labile molecules А3, for example, ozone is possible also. It depends on number of electronic orbits of atom.
The strength A2 will depend on amount of "coils" (electronic orbits) in toroidal "winding" А. If atoms A have on one electron, the connection is implemented on version of a two-electronic bond (fig. 17.3.1). If the atoms A have till two electrons, connection on version of fig. 17.3.1 to be implemented any more can not, and on version of fig. 17.5.1 is even very feeble. At increase of number of electronic orbits in "winding" of a torus, the strength of toroidal connection will at first practically linearly is incremented, and then it is sharply to drop, as the magnetic field is focused inside "winding" and out does not enter almost. Therefore for noble gases the formation of molecules A2 is impossible neither on the mechanism of fig. 17.3.1, nor on the mechanism of fig. 17.5.1. By way of illustration, binding energies (in a kkal/mol) elements forms the first toroidal electronic shell are given below: Li2=25, Be2=0?, B2=69, C2=150, N2=225, O2=118, F2=36, Ne2=0. Feature of chemical compounds with toroidal connection is the absence of electrical dipole moment of molecules. Simultaneously here it is necessary to point; that the circumscribed toroidal connection eliminates an inconsistency arises by quantum mechanical viewing of the molecule О2. "There is one case, in which one a simple method VB (valence bonds - V.K.) conducts to a qualitatively incorrect prediction of an electronic constitution, - the molecule О2. The atom of oxygen in a ground state has a configuration 1S22S22px22py2pz, and consequently it is possible to expect, that two atoms will be joined, forming two two-electronic bonds. Really, for a molecule О2 the binding energy indicates a binary bond, but this molecule has also two not paired electrons, i.e. such combination of the factors, which one is difficult to interpret and even more difficult to forecast, being rest on a method of valence bonds". F. Cotton, G. Wilkinson "Modern inorganic chemistry", "World", М., 1969, 1 part, page 81.
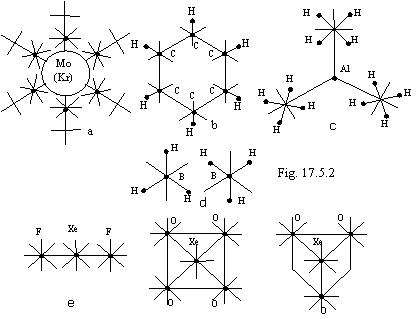
On a figure 17.5.2 the examples of some interesting chemical compounds from a point of view of the parsed types of connections are given.
Mo(CO)6. Octahedron (Fig. 17.5.2a). The affixing of an ion CO- happens at the expense of four electrons interior of a quasicircular orbit and two electrons of the fourth torus Мо. Thus there is an electronic structure Kr and the further extraction of electrons on connection is impossible.
C6H6. Benzol (Fig. 17.5.2b). All connections С-С are equivalent.
Al[BH4]3. A hydride complex (Fig. 17.5.2c). BH4- - tetrahedron.
B2H6. (Fig. 17.5.2d). Two molecules BH3 in that standing, in which one are figured, overlap against each other and are retained at the expense of toroidal connection.
Compounds with noble gases (magnetic connection, Fig. 17.5.2e): XeF2, XeO4 (tetrahedron) and XeO3 (trigonal pyramid).
 |
As it is visible from the schemes of a constitution of molecules, the majority they have not of magnet moment because of complete symmetry of an electron motion. Therefore magnet moment of molecules is determined in the basic of magnet moments of nuclei. The modern physics explains the indicated fact by the zero orbital and spin moment.