16. ON IONIZATION POTENTIALS of ATOMS
In the tables of experimental values of potentials of ionization a lot of information on a constitution of atoms is enciphered, but, while, this information will not be utilized rather effectively because of absence of constitutive ideas. Knowledge, which one we have obtained from the previous chapters could clear up this problem (qualitatively, these tables are already clear and in them there is no "by abnormal" numerals), but the business strongly is complicated by that the binding energy of a given electron with a nucleus includes not only electrostatic interaction, but also interaction of a given electron with all other electrons and simultaneously effective magnetic interaction. Besides at removal of an electron there is a reorganization of all electronic structure of atom. At the same structure of electrons, with increase of nuclear charge the eccentricity of orbits of electrons, as we have found out on an example of helium, drops at first sharply, then slowly. It gives in strengthening connection of an electron with a nucleus at the expense more near-circular orbits on the one hand, and to abatement of this connection at the expense of the greater interaction with other electrons, on the other hand. We have an analytical expression only for one of three simultaneously of varying parameters - binding energy of a given electron with a nucleus, which one of the theory of hydrogen-like atoms can be noted so:
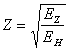 (16.1),
(16.1),
where: EZ - potential of ionization of a hydrogen-like atom with nuclear charge Z, and EH - potential of an atomic ionization of hydrogen. Apparently, that here we have that case, when without good mathematical idea to decide a problem about potentials of ionization of atoms it is impossible. And the idea is encompass following (by the way, it has blanket character and can be utilized for a wide range of similar problems). Let's enter into the formula (16.1) concepts of an effective charge:
Zeff=AZ (16.2),
where A reflects combined effect of interaction of electrons among themselves and magnetic interaction. Let's substitute (16.2) in (16.1) and is conversed to a view:
![]() (16.3),
(16.3),
where: EMn - n-th potential of ionization М - similar of atom, EM1 - first potential of ionization of M-similar atom, Z - charge of an ion, which one will be formed at removal of a given electron, A - parameter depending on a constitution of electronic shells of M-similar atom.
The formula (16.3) will be valid, at A=const,
only at Z![]() , since
the shape of orbits of electrons depends from Z, especially at small Z.
More often in the tables give experimental values of the first ten potentials
of ionization and even it completely insufficiently to compute a precise
parameter value A in the formula (16.3). To decide this problem, we
shall describe experimental values of potentials of ionization М-similar of atoms by any empirical expression, but with
indispensable by a requirement, that it at Z
, since
the shape of orbits of electrons depends from Z, especially at small Z.
More often in the tables give experimental values of the first ten potentials
of ionization and even it completely insufficiently to compute a precise
parameter value A in the formula (16.3). To decide this problem, we
shall describe experimental values of potentials of ionization М-similar of atoms by any empirical expression, but with
indispensable by a requirement, that it at Z![]() gave the formula (16.3). Then it is
not required to know a major series of potentials of ionization and parameter A
it is possible to compute with any precision for anyone М-similar of atom.
gave the formula (16.3). Then it is
not required to know a major series of potentials of ionization and parameter A
it is possible to compute with any precision for anyone М-similar of atom.
For example, for the first three periods of the table of the Mendeleyev I tender following semiempirical dependence (deduction it is not given, since does not introduce interest, parameter B in this dependence no object):
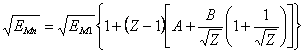 (16.4).
(16.4).
The expression (16.4) at Z![]() gives (16.3), that is an
indispensable requirement.
gives (16.3), that is an
indispensable requirement.
For boron-like of atoms (as an example), in (16.4): А=0.63406, В=0.06633. The matching of experimental values of ionization energy with calculation on (16.4) is given in table 16.1. Table 16.1.
|
Element |
H |
He |
Li |
Be |
B |
|
A |
1.00000 |
0.74271 |
0.78910 |
0.60122 |
0.63406 |
|
Element |
C |
N |
O |
F |
Ne |
|
A |
0.54574 |
0.48029 |
0.49382 |
0.43789 |
0.39704 |
|
Element |
Na |
Mg |
Al |
Si |
P |
|
A |
0.54411 |
0.45078 |
0.50804 |
0.44081 |
0.39181 |
|
Element |
S |
Cl |
Ar |
|
|
|
A |
0.39043 |
0.35119 |
0.31984 |
|
|
|
|
|
|
|
|
|
|
Boron-like atom |
C+1 |
N+2 |
O+3 |
F+4 |
Ne+5
|
|
Z |
2 |
3 |
4 |
5 |
6 |
|
Е exp. (eV) |
24.376 |
47.426 |
77.39 |
114.21 |
157.9 |
|
Е on (57), eV |
24.376 |
47.350 |
77.25 |
114.06 |
157.8 |
|
Boron-like atom |
Na+6 |
Mg+7 |
Al+8 |
Si+9
|
|
|
Z |
7 |
8 |
9 |
10 |
|
|
Е exp. (eV) |
208.44 |
256.84 |
330.1 |
401.3 |
|
|
Е on (57), eV |
208.37 |
256.83 |
330.1 |
401.3 |
|
Table 16.2.
The parameter values A for elements of the first three periods are given in table 16.2.
As the error does not exceed 0.2 %, we shall consider expression (16.4) satisfactory for practical use.
For all remaining elements the expression (16.4) does not allow any more enough precisely to compute parameter A because completely of other constitution of shells (see tab. 15.1) and other empirical expression is required, which one us now to interest will not be, as the principle is clear.
Because of that parameter A is liberated from influence of interaction of an electron with a nucleus and its value does not depend on a constitution М-similar
of atom (including from reorganization of electronic structure at removal of a given electron), apparently, that the electrons forms the same shell of atom and which are taking place from its nucleus on same distance should have and identical ionization energy E0:
![]() (16.5).
(16.5).
It is clearly, that in the first short period E0=13.595 eV, i.e. is peer to ionization energy of atom of hydrogen. Really, for helium: E0 = EHe1×A2 = 24.58/0.742712 = 13.559 eV, therefore value 24.58 – 13.559 = 11.021 eV is stipulated, in basic, magnetic interaction of two electrons in atom of helium (if not to take into consideration gravidynamic interaction). For elements of the second period E0=3.3535 eV, and third period E0=1.5771 eV. Substituting these values in (16.5), we shall discover the first potentials of ionization of these elements; they are shown in table 16.3.
|
Element |
Li |
Be |
B |
C |
N |
|
Е exp. (eV) |
5.39 |
9.32 |
8.296 |
11.264 |
14.54 |
|
Е on (16.5), (eV) |
5.39 |
9.28 |
8.341 |
11.259 |
14.54 |
|
Element |
O |
F |
Ne |
Na |
Mg |
|
Е exp. (eV) |
13.614 |
17.418 |
21.559 |
5.138 |
7.644 |
|
Е on (16.5), (eV) |
13.752 |
17.493 |
21.273 |
5.327 |
7.761 |
|
Element |
Al |
Si |
P |
S |
Cl |
|
Е exp. (eV) |
5.984 |
8.149 |
10.55 |
10.357 |
13.01 |
|
Е on (16.5), (eV) |
6.110 |
8.116 |
10.27 |
10.346 |
12.79 |
|
Element |
Ar |
|
|||
|
Е exp. (eV) |
15.755 |
|
|||
|
Е on (16.5), (eV) |
15.416 |
|
|||
Table 16.3
Structural parameter A completely correlates with that electronic constitution of atoms, which one we have established earlier. As well as it was necessary to expect, the constitution of atom completely determines energy of connections of electrons in its composition. Shell arrangement of electrons around of a nucleus was confirmed. Is shown the essential influence of magnetic interaction to electron-binding energy with atom (in which one is included and gravidynamic interaction, but it in this case is insignificant). The experimental potentials of ionization completely correspond to a constitution of atoms enunciated in this book.
16.1. Ion structure
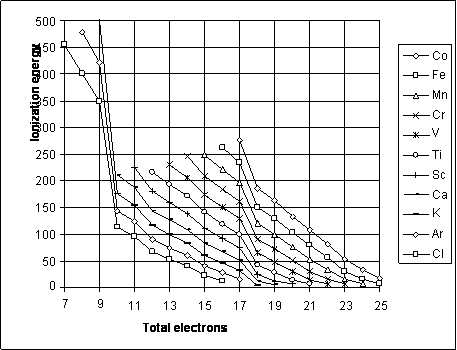
On a figure 16.1.1 the relation of an ionization energy of ions of different elements to total of electrons, inherings to an ion is shown. At 10 electrons the electronic configuration of ions corresponds to a neon, and at 18 electrons - argon. Therefore at the subsequent ionization of such ions the sharp increase of an ionization energy is watched, since it is necessary to shatter filled electronic shell of inert gases. The similarity of curves of a fig. 16.1.1 demonstrates a similarity of electronic structure of the applicable ions. Here only it is necessary to add, that though the electronic structures of ions with the same total number of electrons are similar, but electrons are arranged much closer to a nucleus for multicharge ions, that is quite natural.
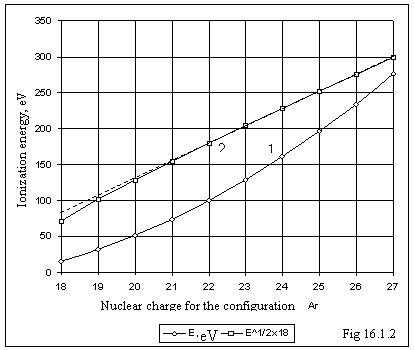
 In a fig. 16.1.2 for the electronic
configuration of ions applicable to argon, the ionization energies E are
adduced depending on nuclear charge of an ion (curve 1) and same values which
have been counted up on the formula
In a fig. 16.1.2 for the electronic
configuration of ions applicable to argon, the ionization energies E are
adduced depending on nuclear charge of an ion (curve 1) and same values which
have been counted up on the formula ![]() (curve 2). In matching from a dashed straight line it is
visible, that with increase of nuclear charge at the same configuration of
electrons the ionization energy is proportional to a square of nuclear charge.
The more charge of an ion, the more precisely is executed quadratic relation,
i.e. the interplay of electrons among themselves in
(curve 2). In matching from a dashed straight line it is
visible, that with increase of nuclear charge at the same configuration of
electrons the ionization energy is proportional to a square of nuclear charge.
The more charge of an ion, the more precisely is executed quadratic relation,
i.e. the interplay of electrons among themselves in
 |
this case can be fling aside.
 |
To receive a spectral line in optical range, the loss of exuberant energy of an electron, order 5-10 eV is necessary. At the same time, the electron-binding energy in an ion Fe+13 beaming a green line «coronium» in a spectrum of the Sun makes approximately 450 eV. This ion has 13 electrons and their configuration is look-alike to the electronic configuration of aluminum. The eccentricity of orbit of an exited electron is peer to ratio of exuberant energy to bond energy. This eccentricity is approximately peer a considered case 0.015. Orbits with such small eccentricity are near to a ground state (circular orbit) therefore metastable - electrons the much greater time for transition in a ground state is necessary. In similar cases we can watch «forbidden» (in the terms of official physics) spectral lines, if the ion is in very rarefied environment and can not lose exuberant energy at impacts with other particles.
16.2. Calculation of ionization potentials
Who though
once saw spectra of composite atoms, that can sympathize to the astronomers,
which one are compelled to be disassembled in thousand spectral lines not only
given element, but also in their mixture with other elements, as it is
substantially watched in space objects. In outcome before eyes of the explorer
there is such bar code in which one practically it is impossible to be
disassembled. Let's presume, that we have a set of spectra of all suspected
elements existing on investigated object. Then the modern computer equipment
can section a spectrum of a complex mixture into spectra of separate elements.
But here there is one more severe difficulty: we do not know spectra of
multicharge ions. For example, in a corona of the Sun the bright green line of
ions Fe+13 (it is watched assigned to a new element «coronium»). And
what all spectrum of this ion and similar to it? It cannot be played back in laboratory
conditions, and to make idealized calculation, it is necessary, at least to
know potentials of ionization of transition Fe+12![]()
![]() Fe+13
Fe+13![]() Fe+14, which one also
cannot be defined experimentally. The given chapter will help to leave from
this desperate situation and to calculate potentials of ionization of any
multicharge ions with a high accuracy.
Fe+14, which one also
cannot be defined experimentally. The given chapter will help to leave from
this desperate situation and to calculate potentials of ionization of any
multicharge ions with a high accuracy.
New physics introduces formation of atomic spectra thus. All electrons of atom are in a ground state and nothing beam. For each electron this condition is strict individually. If atom properly to jolt, the obtained energy is reallocated between all electrons and they will take everyone the personal exited state. At returning in a ground state each electron will beam some serials of spectral lines, number of lines in each serial, basically, is indefinite. Only limit of each serial indicates, that the electron again has taken a ground state. At increase of nuclear charge density of power condition near to a ground state is augmented, therefore spacing interval between spectral lines changes. But as though electron-binding energy with a nucleus was not great, near to a ground state it will beam photons optical and infra-red diapason. On the basis of the set up mechanism of formation of atomic spectra there is a understandable occurrence of spectra, inclusive many thousand of lines.
In chapter 16 the empirical-formula dependences for calculation of potentials of ionization are adduced, but they cannot be recognized satisfactory. In chapter 16.1 (the figure 16.1.1) is shown, that of ions structure with identical number of electrons is look-alike, and on a figure 16.1.2 is shown, what the ionization energy in a degree 1/2 begin with Z+5 and is higher practically linearly depends on nuclear charge (at the same number of electrons). At a charge < Z+5 interplays of electrons among themselves (magnetic and electrostatic) are reduces a potential of ionization, as far as the potential of ionization in each particular case decreases it is impossible to count up, as it is impossible to decide a many-body problem. At rather large nuclear charge the interplay of electrons among themselves practically does not influence interplay with a nucleus, therefore function E(ion)^1/2 ~ Z becomes linear.
Поэтому расчетная формула будет №14: E14=(1,302Z-14,783)2. Мы в эту формулу должны подставить Z=26. В результате получим искомый потенциал ионизации 363,63 эв. Для иона Fe+14 аналогичные расчеты по формуле 12 дадут 460,92 эв.
The author, using the data «the reference Book of the chemist», т.1, 1963, page 325-327 was not too lazy to compound computational equations for all of elements, the data on an ionization energy (eV) which one are accessible. The outcomes are shown in table 16.2.1. In the first column - character of an element, in the second column - nuclear charge of this element, in the third column - formula for calculation of an ionization energy of any ions, which one contain quantity of electrons, equal number of the formula (and only this quantity!), in the subsequent columns matching experimental value of an ionization energy of ions with calculation under the indicated formula is resulted. For example, we shall count up an ionization energy of an ion Fe+12. The nuclear charge iron 26, in the indicated ion is contained 26-12=14 electrons. Therefore this ion attributes to Si-similar atoms (by analogy with hydrogen-like atoms inclusive one electron). Therefore calculated formula will be №14: E14=(1.302Z-14.783)2. We in this formula should substitute Z=26. In outcome we shall receive a required potential of ionization 363.63 eV. For an ion Fe+14 the similar calculations under the formula 12 will give 460.92 eV.
Table 16.2.1.
|
Symbol of element |
Nuclear charge Z
|
Calculated formula |
Nuclear charge |
Z+4 |
Z+5 |
Z+6
|
Z+7
|
Z+8
|
Z+9
|
|
H |
1 |
E1=(3.688Z)2 |
Е(experiment) |
340.03 |
489.65 |
666.47 |
870.49 |
1101.71 |
1360.13 |
|
Е(calculation) |
340.03 |
489.65 |
666.47 |
870.49 |
1101.71 |
1360.13 |
|||
|
He |
2 |
E2=(3.701Z-2.441)2
|
Е(experiment) |
391.99 |
551.93 |
739.11 |
953.8 |
1195.4 |
1464.7 |
|
Е(calculation) |
390.65 |
550.65 |
738.05 |
952.83 |
1195 |
1454.5 |
|||
|
Li |
3 |
E3=(1.852Z-3.062)2
|
Е(experiment) |
97.86 |
138.08 |
185.14 |
239.1 |
299.7 |
367.2 |
|
Е(calculation) |
98.05 |
138.16 |
185.12 |
238.95 |
299.64 |
367.18 |
|||
|
Be |
4 |
E4=(1.857Z-4.182)2
|
Е(experiment) |
113.87 |
157.12 |
207.2 |
264.2 |
328 |
398.6 |
|
Е(calculation) |
113.93 |
157.03 |
207.01 |
263.9 |
327.68 |
398.36 |
|||
|
B |
5 |
E5=(1.869Z-6.134)2
|
Е(experiment) |
114.21 |
157.9 |
208.44 |
265.84 |
330.1 |
401.3 |
|
Е(calculation) |
114.21 |
157.65 |
208.08 |
265.49 |
329.89 |
401.28 |
|||
|
C |
6 |
E6=(1.881Z-7.59)2
|
Е(experiment) |
126.4 |
172.4 |
225.3 |
285.13 |
351.8 |
425.4 |
|
Е(calculation) |
125.89 |
171.64 |
224.46 |
284.36 |
351.34 |
425.39 |
|||
|
N |
7 |
E7=(1.881Z-8.918)2
|
Е(experiment) |
138.6 |
186.8 |
241.8 |
304 |
372.8 |
448.5 |
|
Е(calculation) |
138.6 |
186.43 |
241.34 |
303.32 |
372.37 |
448.51 |
|||
|
O |
8 |
E8=(1.891Z-10.806)2
|
Е(experiment) |
141.23 |
190.42 |
246.41 |
309.3 |
378.9 |
455.3 |
|
Е(calculation) |
141.28 |
189.8 |
245.49 |
308.32 |
378.3 |
455.44 |
|||
|
F |
9 |
E9=(1.905Z-12.404)2
|
Е(experiment) |
153.8 |
205.1 |
263.3 |
328.4 |
400.3 |
479 |
|
Е(calculation) |
152.79 |
203.52 |
261.5 |
326.74 |
399.24 |
479 |
|||
|
Ne |
10 |
E10=(1.915Z-13.94)2
|
Е(experiment) |
166.73 |
220.41 |
280.99 |
348.5 |
422.6 |
503.8 |
|
Е(calculation) |
165.64 |
218.6 |
378.89 |
346.52 |
421.48 |
503.78 |
|||
|
Na |
11 |
E11=(1.303Z-11.524)2
|
Е(experiment) |
65.01 |
88 |
114.2 |
143.4 |
176 |
211.3 |
|
Е(calculation) |
64.34 |
86.94 |
112.93 |
142.32 |
175.11 |
211.29 |
|||
|
Mg |
12 |
E12=(1.296Z-12.227)2
|
Е(experiment) |
72.5 |
96.6 |
123.9 |
154.3 |
187.9 |
224.9 |
|
Е(calculation) |
72.4 |
96.14 |
123.23 |
153.69 |
187.5 |
224.67 |
|||
|
Al |
13 |
E13=(1.293Z-13.719)2
|
Е(experiment) |
67.8 |
91.3 |
117.9 |
143.3 |
180.2 |
216.9 |
|
Е(calculation) |
68.26 |
91.3 |
117.68 |
147.4 |
180.47 |
216.88 |
|||
|
Si |
14 |
E14=(1.302Z-14.783)2
|
Е(experiment) |
75 |
99.4 |
127.9 |
159.2 |
193.1 |
230.2 |
|
Е(calculation) |
74,87 |
99.1 |
126.72 |
157.73 |
192.13 |
229.92 |
|||
|
P |
15 |
E15=(1.319Z-15.977)2
|
Е(experiment) |
82.6 |
109 |
139 |
172 |
206 |
246 |
|
Е(calculation) |
82.52 |
108.22 |
137.4 |
170.07 |
206.21 |
245.83 |
|||
|
S |
16 |
E16=(1.311Z-16.995)2
|
Е(experiment) |
84 |
111 |
141 |
174 |
209 |
249 |
|
Е(calculation) |
85.1 |
111 |
140.35 |
173.13 |
209.35 |
249 |
|||
|
Cl |
17 |
E17=(1.321Z-18.16)2
|
Е(experiment) |
91.8 |
119 |
151 |
185 |
221 |
262 |
|
Е(calculation) |
91.8 |
118.85 |
149.4 |
183.44 |
220.97 |
261.99 |
|||
|
Ar |
18 |
E18=(1.33Z-19.27)2
|
Е(experiment) |
99.8 |
128.9 |
161.1 |
196.4 |
234.4 |
276.9 |
|
Е(calculation) |
99.8 |
128.14 |
160.02 |
195.44 |
234.4 |
276.89 |
|||
|
K |
19 |
E19=(1.362Z-23.176)2
|
Е(experiment) |
65.2 |
90.6 |
120 |
151 |
185.9 |
224 |
|
Е(calculation) |
66.42 |
90.48 |
118.24 |
149.72 |
184.91 |
223.8 |
|||
|
Ca |
20 |
E20=(1.381-24.525)2
|
Е(experiment) |
73 |
100 |
130 |
163 |
200 |
241 |
|
Е(calculation) |
74.29 |
100 |
129.53 |
162.87 |
200.02 |
240.99 |
|||
|
Sc |
21 |
E21=(1.4Z-26.272)2
|
Е(experiment) |
76 |
103 |
133 |
168 |
206 |
247 |
|
Е(calculation) |
76.18 |
102.58 |
132.89 |
167.13 |
205.29 |
247.37 |
|||
|
Ti |
22 |
E22=(1.515Z-30.497)2
|
Е(experiment) |
79 |
109 |
143 |
182 |
224 |
271 |
|
Е(calculation) |
79.08 |
108.33 |
142.16 |
180.58 |
223.59 |
271.19 |
|||
|
V |
23 |
E23=(1.542Z-32.601)2
|
Е(experiment) |
82 |
113 |
148 |
188 |
231 |
280 |
|
Е(calculation) |
81.59 |
111.83 |
146.82 |
186.57 |
231.07 |
280.33 |
|||
|
Cr |
24 |
E24=(1.528Z-33.879)2
|
Е(experiment) |
79 |
109 |
144 |
183 |
226 |
274 |
|
Е(calculation) |
79.3 |
108.85 |
143.06 |
181.95 |
225.51 |
273.74 |
|||
|
Mn |
25 |
E25=(1.529Z-35.193)2
|
Е(experiment) |
83 |
114 |
149 |
189 |
234 |
282 |
|
Е(calculation) |
83.69 |
114 |
148.99 |
188.65 |
232.99 |
282 |
|||
|
Fe |
26 |
E26=(1.549Z-37.156)2
|
Е(experiment) |
86 |
118 |
155 |
196 |
241 |
291 |
|
Е(calculation) |
86.75 |
118 |
154.06 |
194.91 |
240.56 |
291 |
|||
|
Co |
27 |
E27=(1.557Z-38.75)2
|
Е(experiment) |
90 |
123 |
160 |
202 |
248 |
300 |
|
Е(calculation) |
90.57 |
122.63 |
159.54 |
201.3 |
247.9 |
299.36 |
|||
|
Ni |
28 |
E28=(1.338Z-32.854)2
|
Е(experiment) |
93.4 |
127.5 |
155 |
193 |
234 |
277 |
|
Е(calculation) |
99.24 |
127.69 |
159.72 |
195.33 |
234.52 |
277.29 |
|||
|
Cu |
29 |
E29=(1.061Z-27)2
|
Е(experiment) |
62.9 |
82.1 |
103 |
126 |
150 |
177 |
|
Е(calculation) |
64.21 |
82.34 |
102.72 |
125.35 |
150.23 |
177.37 |
|||
|
Zn |
30 |
E30=(1.102Z-29.148)2
|
Е(experiment) |
68.3 |
88.6 |
111 |
136 |
162 |
191 |
|
Е(calculation) |
69.22 |
88.77 |
110.75 |
135.16 |
162 |
191.27 |
|||
|
Ga |
31 |
E31=(1.073Z-29.767)2
|
Е(experiment) |
59.7 |
78.5 |
99.2 |
122.3 |
146.2 |
173 |
|
Е(calculation) |
60.65 |
78.52 |
98.68 |
121.15 |
145.93 |
173 |
|||
|
Ge |
32 |
E32=(1.119Z-32.233)2
|
Е(experiment) |
64.7 |
84.4 |
106 |
129 |
154 |
186 |
|
Е(calculation) |
64.82 |
84.09 |
105.86 |
130.14 |
156.93 |
186.21 |
|||
|
As |
33 |
E33=(1.053Z-30.298)2
|
Е(experiment) |
71 |
90.8 |
116 |
139 |
165 |
194 |
|
Е(calculation) |
75.05 |
94.4 |
115.97 |
139.76 |
165.77 |
193.99 |
|||
|
Se |
34 |
E34=(1.08Z-32.476)2
|
Е(experiment) |
71.6 |
93 |
116 |
141 |
167 |
195 |
|
Е(calculation) |
73.34 |
93.01 |
115 |
139.33 |
166 |
195 |
|||
|
Br |
35 |
E35=(1.19Z-37.663)2
|
Е(experiment) |
77 |
99.4 |
124 |
153 |
183 |
216 |
|
Е(calculation) |
76.51 |
98.74 |
123.81 |
151.71 |
182.44 |
216 |
|||
|
Kr |
36 |
E36=(1.123Z-35.535)2
|
Е(experiment) |
82.3 |
110.4 |
131 |
161 |
192 |
225 |
|
Е(calculation) |
88.08 |
110.42 |
135.28 |
162.66 |
192.57 |
225 |
|||
|
Rb |
37 |
E37=(1.252Z-44.25)2
|
Е(experiment) |
50 |
67 |
94 |
119 |
147 |
178 |
|
Е(calculation) |
50.15 |
69.45 |
91.89 |
117.46 |
146.17 |
178 |
|||
|
Sr |
38 |
E38=(1.239Z-44.558)2
|
Е(experiment) |
61.2 |
76 |
100 |
126 |
155 |
187 |
|
Е(calculation) |
55.95 |
76.02 |
99.16 |
125.37 |
154.65 |
187.01 |
|||
|
Y |
39 |
E39=(1.238Z-45.46)2
|
Е(experiment) |
59 |
81 |
105 |
132 |
162 |
195 |
|
Е(calculation) |
60.43 |
81.22 |
105.06 |
131.97 |
161.95 |
194.99 |
|||
|
Zr |
40 |
E40=(1.263Z-47.604)2
|
Е(experiment) |
63 |
85 |
111 |
139 |
170 |
204 |
|
Е(calculation) |
63.49 |
85.21 |
110.12 |
138.23 |
169.52 |
204 |
|||
|
Nb |
41 |
E41=(1.277Z-49.256)2
|
Е(experiment) |
67 |
90 |
116 |
146 |
178 |
213 |
|
Е(calculation) |
67.39 |
89.98 |
115.84 |
144.96 |
177.34 |
212.98 |
|||
|
Mo |
42 |
E42=(1.28Z-50.754)2
|
Е(experiment) |
66 |
89 |
115 |
144 |
176 |
211 |
|
Е(calculation) |
66.03 |
88.47 |
114.19 |
143.18 |
175.46 |
211 |
|||
|
Tc |
43 |
E43=(1.293Z-52.404)2
|
Е(experiment) |
70 |
94 |
121 |
151 |
184 |
220 |
|
Е(calculation) |
70 |
93.32 |
119.97 |
149.96 |
183.3 |
219.99 |
|||
|
Ru |
44 |
E44=(1.308Z-54.191)2
|
Е(experiment) |
73 |
98 |
126 |
157 |
192 |
229 |
|
Е(calculation) |
73.84 |
98.03 |
125.64 |
156.67 |
191.13 |
229.01 |
|||
|
Rh |
45 |
E45=(1.335Z-56.663)2
|
Е(experiment) |
77 |
103 |
132 |
164 |
200 |
238 |
|
Е(calculation) |
76.6 |
101.75 |
130.46 |
162.74 |
198.58 |
237.99 |
|||
|
Pd |
46 |
E46=(1.273Z-54.015)2
|
Е(experiment) |
91 |
119 |
149 |
182 |
218 |
256 |
|
Е(calculation) |
92.83 |
116.98 |
148.38 |
181.01 |
216.88 |
256 |
|||
|
Ag |
47 |
E47=(1.065Z-46.328)2
|
Е(experiment) |
63.8 |
83 |
104 |
126 |
150 |
158 |
|
Е(calculation) |
63.79 |
81.94 |
102.35 |
125.04 |
150 |
177.21 |
|||
|
Cd |
48 |
E48=(0.944Z-40.963)2
|
Е(experiment) |
66 |
83 |
102 |
122 |
144 |
165 |
|
Е(calculation) |
66.02 |
82.25 |
100.26 |
120.06 |
141.63 |
164.99 |
|||
|
In |
49 |
E49=(0.938Z-41.289)2
|
Е(experiment) |
71 |
89 |
108 |
127 |
151 |
172 |
|
Е(calculation) |
70.98 |
87.67 |
106.11 |
126.31 |
148.28 |
172 |
|||
|
Sn |
50 |
E50=(1.023Z-47.629)2
|
Е(experiment) |
57 |
74 |
93 |
114 |
137 |
162 |
|
Е(calculation) |
57.96 |
74.58 |
93.3 |
114.1 |
137.01 |
162 |
|||
|
Sb |
51 |
E51=(1.041Z-49.383)2
|
Е(experiment) |
62 |
80 |
100 |
122 |
146 |
171 |
|
Е(calculation) |
61.97 |
79.44 |
99.08 |
120.89 |
144.86 |
171.01 |
|||
|
Te |
52 |
E52=(1.056Z-51.263)2
|
Е(experiment) |
62 |
80 |
100 |
122 |
147 |
173 |
|
Е(calculation) |
61.98 |
79.73 |
99.7 |
121.9 |
146.34 |
173 |
|||
|
J |
53 |
E53=(1.066Z-52.638)2
|
Е(experiment) |
66 |
85 |
106 |
129 |
154 |
181 |
|
Е(calculation) |
66 |
84.46 |
105.18 |
128.19 |
153.46 |
181.01 |
|||
|
Xe |
54 |
E54=(1.06Z-53.105)2
|
Е(experiment) |
70 |
89 |
111 |
135 |
161 |
187 |
|
Е(calculation) |
70.14 |
89.02 |
110.14 |
133.52 |
159.14 |
187.01 |
|||
|
NO DATA |
Е(experiment) |
|
|
|
|
|
|
||
|
Е(calculation) |
|
|
|
|
|
|
|||
|
Tu |
69 |
E69=(1.075Z-71.767)2
|
Е(experiment) |
45 |
61 |
79 |
99 |
121 |
146 |
|
Е(calculation) |
45 |
60.57 |
78.46 |
98.66 |
121.18 |
146 |
|||
|
Yb |
70 |
E70=(1.088Z-73.583)2
|
Е(experiment) |
48 |
65 |
89 |
104 |
127 |
153 |
|
Е(calculation) |
48.01 |
64.27 |
82.9 |
103.9 |
127.26 |
152.99 |
|||
|
Lu |
71 |
E71=(1.091Z-74.671)2
|
Е(experiment) |
51 |
68 |
88 |
109 |
133 |
159 |
|
Е(calculation) |
51.18 |
67.98 |
87.16 |
108.72 |
132.66 |
158.99 |
|||
|
Hf |
72 |
E72=(1.1Z-76.216)2
|
Е(experiment) |
54 |
72 |
92 |
114 |
139 |
166 |
|
Е(calculation) |
54.52 |
71.98 |
91.85 |
114.15 |
138.86 |
166 |
|||
|
Ta |
73 |
E73=(1.123Z-78.933)2
|
Е(experiment) |
57 |
75 |
96 |
120 |
145 |
173 |
|
Е(calculation) |
56.82 |
75.01 |
95.73 |
118.96 |
144.72 |
173 |
|||
|
W |
74 |
E74=(1.126Z-80.458)2
|
Е(experiment) |
55 |
73 |
94 |
117 |
142 |
169 |
|
Е(calculation) |
54.32 |
72.18 |
92.58 |
115.52 |
140.99 |
169 |
|||
|
Re |
75 |
E75=(1.123Z-81.066)2
|
Е(experiment) |
58 |
77 |
98 |
112? |
148 |
176 |
|
Е(calculation) |
58.54 |
76.98 |
97.95 |
121.44 |
147.45 |
175.99 |
|||
|
Os |
76 |
E76=(1.144Z-83.712)2
|
Е(experiment) |
61 |
81 |
103 |
127 |
154 |
183 |
|
Е(calculation) |
60.96 |
80.14 |
101.93 |
126.34 |
153.36 |
183.01 |
|||
|
Ir |
77 |
E77=(1.157Z-85.718)2
|
Е(experiment) |
64 |
84 |
107 |
132 |
160 |
190 |
|
Е(calculation) |
63.98 |
83.83 |
106.36 |
131.56 |
159.44 |
190 |
|||
|
Pt |
78 |
E78=(1.137Z-84.883)2
|
Е(experiment) |
69.7 |
94.4 |
112 |
138 |
166 |
197 |
|
Е(calculation) |
69.74 |
90.02 |
112.89 |
138.34 |
166.38 |
197.01 |
|||
|
Au |
79 |
E79=(Z-75.51)2
|
Е(experiment) |
56 |
73 |
91 |
111 |
133 |
156 |
|
Е(calculation) |
56.1 |
72.08 |
90.06 |
110.04 |
132.02 |
156 |
|||
|
Hg |
80 |
E80=(0.999Z-76.105)2
|
Е(experiment) |
61 |
78 |
97 |
117 |
140 |
164 |
|
Е(calculation) |
61.01 |
77.62 |
96.22 |
116.81 |
139.4 |
163.99 |
|||
|
Tl |
81 |
E81=(0.98Z-76.158)2
|
Е(experiment) |
51 |
67 |
84 |
103 |
123 |
145 |
|
Е(calculation) |
51.01 |
65.97 |
82.85 |
101.65 |
122.37 |
145.01 |
|||
|
Pb |
82 |
E82=(0.996Z-78.226)2
|
Е(experiment) |
55 |
71 |
89 |
109 |
130 |
154 |
|
Е(calculation) |
55.2 |
71 |
88.77 |
108.53 |
130.28 |
154.01 |
|||
|
Bi |
83 |
E83=(1.01Z-80.192)2
|
Е(experiment) |
59 |
76 |
95 |
115 |
138 |
162 |
|
Е(calculation) |
58.95 |
75.48 |
94.05 |
114.66 |
137.31 |
162 |
|||
|
Po |
84 |
E84=(1.01Z-81.215)2
|
Е(experiment) |
59 |
76 |
94 |
115 |
137 |
? |
|
Е(calculation) |
58.75 |
75.26 |
93.8 |
114.38 |
137.01 |
? |
|||
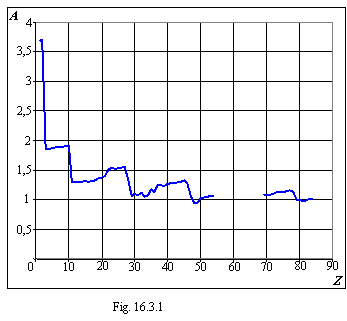
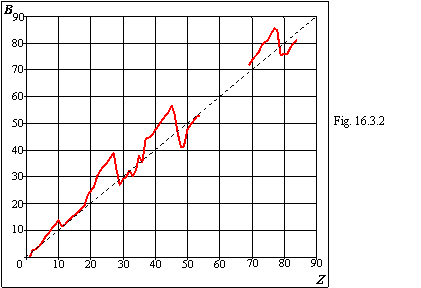
16.3 Values of coefficients in the formulas of an ionization energy
Values of coefficients in the formulas of an ionization energy of a kind EZ=(AZ-B)2 of chapter 16.2.
On a figure 16.3.1 the course of change of a factor A is shown depending on nuclear charge, and on a figure 16.3.2 - similar course of change of a factor B. As the factor B, on the average, is proportional to nuclear charge, on a figure 16.3.2 the broken line of direct ratio is conducted. From table 16.3.1 and graphs the correlation of factors A and B with degree of fullness of electronic envelopes and subenvelopes of table 15.1 of chapter 15 is legiblly visible and it proves the new form of the table of the Mendeleyev.
|
Z at E numerically is equal to number of the formula of chapter 16.2, which one is peer to nuclear charge of this element (Z brackets) minus a charge of any ion of this element. Z |
A |
B |
Z |
A |
B |
Z |
A |
B |
Comment |
|
1 |
3,688 |
0,000 |
25 |
1,529 |
35,193 |
49 |
0,938 |
41,289 |
In brackets the number of electrons in a envelope or sub envelope of table 15.1 is indicated |
|
2 |
3,701 (2) |
2,441 |
26 |
1,549 |
37,156 |
50 |
1,023 |
47,629 |
|
|
3 |
1,852 |
3,062 |
27 |
1,557 |
38,750 |
51 |
1,041 |
49,383 |
|
|
4 |
1,857 |
4,182 |
28 |
1,338 (8) |
32,854 |
52 |
1,056 |
51,263 |
|
|
5 |
1,869 |
6,134 |
29 |
1,061 |
27,000 |
53 |
1,066 |
52,638 |
|
|
6 |
1,881 |
7,590 |
30 |
1,102 (2) |
29,148 |
54 |
1,060 (6) |
53,105 |
For elements with z=55 up to z=68 there are no data |
|
7 |
1,881 |
8,918 |
31 |
1,073 |
29,767 |
69 |
1,070 |
71,767 |
|
|
8 |
1,891 |
10,806 |
32 |
1,119 |
32,233 |
70 |
1,088 |
73,583 |
|
|
9 |
1,905 |
12,404 |
33 |
1,053 |
30,298 |
71 |
1,091 (16) |
74,871 |
|
|
10 |
1,915 (8) |
13,940 |
34 |
1,080 |
32,476 |
72 |
1,100 |
76,216 |
|
|
11 |
1,303 |
11,524 |
35 |
1,190 |
37,663 |
73 |
1,123 |
78,933 |
|
|
12 |
1,296 |
12,227 |
36 |
1,123 (6) |
35,535 |
74 |
1,126 |
80,458 |
|
|
13 |
1,293 |
13,719 |
37 |
1,252 |
44,250 |
75 |
1,123 |
81,066 |
|
|
14 |
1,302 |
14,783 |
38 |
1,239 (2) |
44,558 |
76 |
1,144 |
83,712 |
|
|
15 |
1,319 |
15,977 |
39 |
1,238 |
45,460 |
77 |
1,157 |
85,718 |
|
|
16 |
1,311 |
16,995 |
40 |
1,263 |
47,604 |
78 |
1,137 |
84,883 |
|
|
17 |
1,321 |
18,160 |
41 |
1,277 |
49,256 |
79 |
1,000 (8) |
75,510 |
|
|
18 |
1,330 (8) |
19,270 |
42 |
1,280 |
50,754 |
80 |
0,999 |
76,105 |
|
|
19 |
1,362 |
23,176 |
43 |
1,293 |
52,404 |
81 |
0,980 (2) |
76,158 |
|
|
20 |
1,381 (2) |
24,525 |
44 |
1,308 |
54,191 |
82 |
0,996 |
78,226 |
|
|
21 |
1,400 |
26,272 |
45 |
1,335 |
56,663 |
83 |
1,010 |
80,192 |
|
|
22 |
1,515 |
30,497 |
46 |
1,273 (8) |
54,015 |
84 |
1,010 |
81,215 |
|
|
23 |
1,542 |
32,601 |
47 |
1,065 |
46,328 |
|
|
|
|
|
24 |
1,528 |
33,879 |
48 |
0,944 (2) |
40,963 |
|
|
|
|
 |
 |