|
|
|
|
|
|
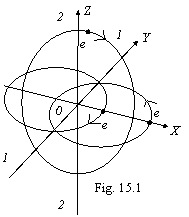
Principle of formation their same - greatest possible winning of energy. For three-electronic
atoms the possible invariable structure is single will be then, when the third
electron will have an orbit in a plane, perpendicular plane of orbits of two
electrons of helium-like atom, that are show on a figure 15.1 (lithium).
The third electron
can not move apart orbits of two first electrons because of their strong
magnetic interaction (see of fig. 15.7). If not influence of two electrons, the
third electron would have a circular orbit, but the influence of these
electrons gives in a tension of an orbit along a line 1-1 and cutting along a
line 2-2, that determines ellipsoidal an orbit with a eccentricity, order e=0.777
for lithium, which one at first it is sharply, and then feebly is incremented
with increase of nuclear charge up to, for example, e=0.854. Thus the
nucleus is at centre of an equivalent ellipse, instead of in its focus.
|
|
|
|
|
|
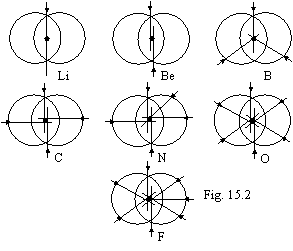
At adding the fourth electron, from the
third and fourth electrons the similarity of system of the first and second
electron, only in a perpendicular plane will be formed, thus the nucleus
appears in focus of ellipsoidal orbits. Further this process is prolonged
before formation of a 8-electronic torus without an interior hole (fig. 15.2)
in which one if to be moves on a circle lengthwise axis of torus, the electrons
in it meet rotary around of a nucleus in one side.
On fig. 15.2 orbits
in para-position as of convenience perceptions are displaced relatively each
other. Aspiration to pairing electrons both between interacting atoms, and at
formation of a torus (fig. 15.2) is stipulated by alone aspiration to a minimum
of a potential energy, which one is ensured, in this case, by interaction of orbital
of magnet moments and makes a basis of chemistry. In this respect there is some
analogy to nucleons in a nucleus, which one in the same way ensure a minimum of
a potential energy own of the gravidynamic moments (see theory of a nucleus).
|
|
|
|
|
|
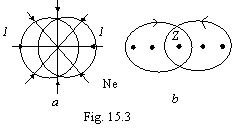
On a figure 15.3 the
neon as by way of on a plane of orbits of two first electrons (a) and in a slit
1-1 (b) is figured. The point on figure 15.3b shown orbits of two first
electrons inside a torus moves in the counter sides.
From a figure 15.2 it
is visible, that, despite of a blanket utility of formation of a torus and
bound with it increase of an ionization energy, the affixing of the third and
sixth electrons of a torus to the greatest degree deforms already existing up
structure, therefore ionization energy for these electrons appears much below
anticipated.
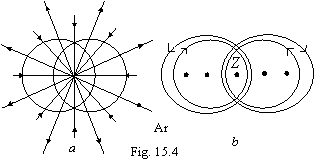
Further process goes in precisely
the same way, thus thin split of spectral lines, for example, sodium, is
stipulated by a miscellaneous current of traffic of an outside electron for
miscellaneous atoms of the same element. In the result as though the double
torus with an opposite electron motion is gained, that are shown on a figure
15.4 (argon).
|
|
|
|
|
|
Here there is a very tempting opportunity (from an energy point of view)
more tight to fill atom by electrons, by arranging them on quasicircular orbits
inside the second torus, but this opportunity cannot be implemented because of
absence of "hole" through which one electrons could get to
destination and, most important - the energy situation has not ripened yet for
embodying this opportunity.
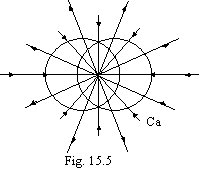
The approaching requirements occur at adding a pair of electrons initial to shape one more outside torus, we shall recollect, that the third electron to build into a torus not so profitably (fig. 15.2, В). It is figured on a figure 15.5 (calcium).
|
|
|
|
|
|
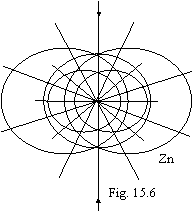
Now becomes profitably to not finish building further third torus, and to fill first
almost by circular orbit on which one 8 electrons (from scandium up to nickel)
place only, and in atoms of cuprum and zinc already the second torus by two
electrons in a standing similar to helium is stuffed. The atom of zinc is
figured on fig. 15.6.
Further is finished building the third torus starting from atom gallium
and finishing a krypton. Then all process is iterated again. In atoms of
rubidium and strontium starts the formation of the fourth torus, start with
yttrium till a palladium 8 electrons on an almost circular orbit stuffs in an
interior of the second torus, in atoms of argent and cadmium is stuffed already
the third torus by two electrons in a standing similar to zinc and from indium
till a xenon formation of the fourth torus is terminated.
Then is exact as in cesium and
barium the beginning of the fifth torus is shaped, start with lanthanum till
gadolinium 8 electrons on an almost circular orbit stuff in an interior of the
third torus.
|
|
|
|
|
|
As the influence of a nucleus is considerably increased, there is an
opportunity to 8 electrons (with terbium till hafnium) to form second an quasicircular
orbit with an opposite electron motion inside the third torus and already not
two electrons, and 8 (from a tantalum till hydrargyrum) stuff in the fourth
torus in a plane of pair orbits of zinc and cadmium. If there were no two
electrons of the fifth torus, 8 elements in a series a tantalum - hydrargyrum
would be a certain similarity of elements in series sodium - argon. Thus, the
hydrargyrum is in some respects look-alike to noble gases, because of what it
represents a fluid in standard conditions. "Except for hydrargyrum, all
maximums on a curve (first potentials of ionization depending on atomic numbers
of elements - V.K.) are watched for noble gases and the more deep minimums for
alkali metals". F. Cotton, G. Wilkinson, Modern inorganic chemistry,
"World", М.,
1969, 1 part, page 47.
Further with thallium till a radon the formation of the fifth torus is terminated, and francium and radium start shaping the sixth torus. 8 elements from actinium on кюрий will forms a quasicircular orbit inside the fourth torus, and 8 elements with berkelium till 104 element - second almost circular orbit inside the fourth torus with an opposite electron motion. In hypothetical elements with 105 till 112 eight electrons would stuff in the fifth torus in a plane of pair orbits and would be chemical analogs of a series a tantalum - hydrargyrum, in elements with 113 on 118 the formation of the sixth torus would be completed. The terminations of this filling and the more so terminating of shaping of the sixth torus we, on all visibility, never shall see because of instability of heavy nuclei, instead of because of any restrictions on the part of electronic shells. Therefore hopes of the scientists on "an island region of stability" in far transuranium elements will not be justified. By the way, now under a shell it is necessary to understand one of 8-electronic toruses inserted each other, as matreshkas, together with their interior content. If to be more precise, exist of a miscellaneous view of a shells in essence distinguished from each other: formatives a torus and lying in a plane, perpendicular axis of a torus. Last, in turn, are subdivided into two and eight-electronic with orbits of electrons similar to orbits in toruses, but lying in one plane and eight and 16-electronic of a quasicircular orbit in the same plane, on which one the electrons are collective. The sizes of all atoms in a nonexcited state
|
|
|
|
|
|
are about identical, switching here and hydrogen. It indicates that all
electrons in atom are on stationary orbits, the size which one wholly is
determined by traveling speed of an electron so that they met condition: one
wave de Broglie, the mechanical moment is peer to the moment of a mobile
electron. In this connection arouses surprise of the logic of a modern physics,
when quantum numbers, in which one there can be an excited atom of hydrogen,
mechanically are transferred on all atoms, considering is completely
groundless, that only these states and are permitted. We have shown that these
states metastable and any relation to a constitution of nonexcited atoms have
not. Really, radius of an orbit of an electron is inversely proportional to
nuclear charge and is directly proportional to a quadrate of a quantum number;
therefore, from a point of view of a modern physics, radius of atoms should
linearly be incremented with increase of nuclear charge. By sets of quantum
numbers it is impossible to describe a constitution of atoms if not to resort
to eliminations, by amount is significant superior rules, of what the curious
reader can personally be convinced, if he will have enough patience to
understand it the finally tangled problem.
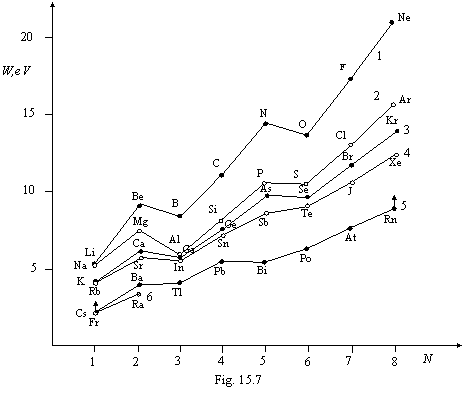
By constructing the diagrams of the
first ionization energy of atom depending on number of electrons formatives a
given torus (of fig. 15.7), we visuals the steady tendency to a rectification
of the diagrams with increase of the number of a torus (for 5-th and 6-th torus
the diagrams are displaced downwards on 2 eV as a matter of convenience
viewings). It is bound to increase of azimuth mobility of electronic orbits
with increase of nuclear charge. Thus in the fifth torus the fifth electron is
already capable to move apart orbits of the previous electrons (we compare from
fig. 15.2N). Nevertheless, the affixing of the third electron remains energy
poorly to turn to advantage, that is visible on a start of the diagram for the
sixth torus, namely, this circumstance and determines rules of build-up of
electronic shells of atoms, which one remain, thus, invariable not only for
existing, but also for hypothetical elements. If it was not so, the filling by
electrons of an interior of the fifth torus could not happen. In due course, we
shall learn to do to profitable affixing of the third electron to a torus and
to that there are some independent paths. Unique opportunities for chemistry in
this case are unclosed, for example, it is possible to receive iron as noble
gas.
Table 15.1
|
Fil ling of toruses |
Filling of quasicircular orbits |
Filling
of orbits in one plane |
|
(H) (He) |
|
(H)(He) |
|
Li
Be B
C N O F Ne |
|
|
|
Na
Mg Al Si P
S Cl Ar |
|
|
|
K Ca |
Sc Ti
V Cr Mn Fe Co Ni |
Cu Zn |
|
Ga Ge As Se Br Kr |
|
|
|
Rb Sr |
Y Zr Nb Mo Tc Ru Rh Pd |
Ag Cd |
|
In Sn Sb Te J Xe |
|
|
|
Cs Ba |
La Ce Pr Nd Pm Sm
Eu Gd
Tb Dy Ho Er Tu Yb Lu Hf |
Ta
W Re Os Ir Pt Au Hg |
|
Tl Pb Bi Po At Rn |
|
|
|
Fr Ra |
Ac Th Pa U
Np Pu Am Cm
Bk Cf Es Fm Md No Lw 104 |
105 106 107 108 109 110 111 112 |
|
113 114 115 116 117 118 |
|
|
In table 15.1 the plan of filling by electrons of toruses, of quasicircular
orbits and orbits in one plane is shown. As a matter of fact, this table
represents the new shape of the table of the D.I. Mendeleyev: the elements on
one vertical have similar chemical properties. "But it, however, does not
mean, what even the perfect knowledge of electronic configurations of atoms in
all without elimination cases allows to do unique deductions concerning
properties of the relevant elements". "About a systematic of
particles", 1969, page 40.
Except for short
"period": H, He, which one takes the special standing,
remaining start with alkali metal and are finished by noble gas. Thus they can
be compared only pairwise, since the constitution of the subsequent pair
differs from previous sharply.
If in a modern physics, which one
considers electrons in atom "spread" in space there is no problem on
sync of their motion, in featured here to neoclassical physics this problem is
major not only for exact comprehension of a constitution of atoms, but also at
formation of molecules from atoms and solid bodies from molecules and in more
detail will be surveyed below. In the same partition we should pay attention
that the sync is possible only for two electrons, the orbits which one lie in
one plane and completely are symmetrical concerning a nucleus or for a lot of
electrons, if they place symmetrically on one to a quasicircular orbit. In the
latter case atoms have magnetic properties. All remaining versions can not be
practically implemented because of impossibility of a synchronous electron
motion. The requirement of sync of electrons has by a self-acting corollary
high symmetry of a constitution of atoms, molecules, solid bodies, as a matter
of fact - high symmetry of all Universes.
The orthodoxes,
especially with a mathematical bias, recognize which one except for the
formulas nothing, can charge the author of only speculative exposition of a
constitution of atoms. On it is possible to object following: speculative
exposition - much more effective method of knowledge when mathematics is powerless.
If the Copernicus instead of speculative exposition of a constitution of a
solar System attempted to make it mathematically, he would be excruciated with
this problem about today and without any result, since such exposition does not
exist now. And try "mathematizing" the theory of evolution of the
Comments of the author to chapter 15:
1. Money to a wind.
Mass media is joy have notified about new success of Russian and
American scientists: six atoms of a chemical element № 117 are obtained. It is
a pity, that do not inform, how much money is consumed for two years for
obtaining these of six atoms. A principle of obtaining of transuranic elements
extreme artless: irradiate a high-gravity element with nuclei of other element
and catch, that was received. Seldom, but sometimes is received that is
necessary. The truth the superhigh-gravity elements so fast are disintegrated,
that occasionally have no time them identify. If to expend it is even more time
and money, it is possible entertain the self-love at the expense of the tax
bearers and to receive 118 element (table 15.1) and so on, but what is it gives
for science? Nothing. Instead of three-storeyed the houses can be expended more
time and money and to construct four-storeyed etc. For building science it too
nothing gives.
The new physics
states, that radius of a motion of an electron in atom is proportional to electronic
mass (see formula 2.3). The official physics holds on to the opposite
statement: distance of an electron from a nucleus of atom is inversely
proportional to electronic mass (see chapter 2). The mesoatoms contain in the
composition instead of an electron a meson (![]() ,
, ![]() ,
, ![]() etc.).
The sizes of mesoatoms are less than the sizes of customary atoms in as much
time, in how many of time mass of mesons is more than electronic mass, i.e.
correspond to the official formula for radius of atom, where the electronic
mass (meson) stands in a denominator. From this fact it would be possible to
draw a conclusion that the deductions of new physics on a given problem are
erroneous, however at more close examination of a problem it appears, that the
error is done by orthodox physics. The conflict consists in following.
Apparently, that the gravitational interaction of an electron with a nucleus
insignificantly also can not influence behavior of an electron. At the same
time, the electronic mass can exhibit itself only as inertia at a motion of an
electron on a certain orbit, incrementing its radius. On notions of a quantum
mechanics the electron in atom has not orbital motion, therefore electronic
mass at all should not figure in the formula for radius, and the occurrence it
in a denominator contradicts physical sense, since except for inertia of any
other functions it to bear can not. To decide the indicated conflict, it is
necessary to pay attention to a moment of momentum on an orbit, which one as a
result of operation of law of maintenance of an angular momentum maintains
constant value: S=mVr. At the same angular momentum of miscellaneous
particles (the new physics demonstrates, that it is peer 1 in terms of h/2
etc.).
The sizes of mesoatoms are less than the sizes of customary atoms in as much
time, in how many of time mass of mesons is more than electronic mass, i.e.
correspond to the official formula for radius of atom, where the electronic
mass (meson) stands in a denominator. From this fact it would be possible to
draw a conclusion that the deductions of new physics on a given problem are
erroneous, however at more close examination of a problem it appears, that the
error is done by orthodox physics. The conflict consists in following.
Apparently, that the gravitational interaction of an electron with a nucleus
insignificantly also can not influence behavior of an electron. At the same
time, the electronic mass can exhibit itself only as inertia at a motion of an
electron on a certain orbit, incrementing its radius. On notions of a quantum
mechanics the electron in atom has not orbital motion, therefore electronic
mass at all should not figure in the formula for radius, and the occurrence it
in a denominator contradicts physical sense, since except for inertia of any
other functions it to bear can not. To decide the indicated conflict, it is
necessary to pay attention to a moment of momentum on an orbit, which one as a
result of operation of law of maintenance of an angular momentum maintains
constant value: S=mVr. At the same angular momentum of miscellaneous
particles (the new physics demonstrates, that it is peer 1 in terms of h/2![]() )
according to activity of this law radius of an orbit of a particle will be
inversely proportional to its mass at the same velocity of orbital motion (this
velocity near to a nucleus comes nearer to speed of light, that is convincingly
shown in chapter 5.1). Thus, physically are just and are valid two outwardly
opposite statements: in the formula for radius of an orbit the particle mass
should stand in numerator from a point of view of inertia of a particle and in
a denominator from a point of view of maintenance of an angular momentum, if we
shall decrypt it, but it cannot be made, since it is a constant. Unfortunately,
the orthodox physics does not understand physical substance it’s of the
formulas.
)
according to activity of this law radius of an orbit of a particle will be
inversely proportional to its mass at the same velocity of orbital motion (this
velocity near to a nucleus comes nearer to speed of light, that is convincingly
shown in chapter 5.1). Thus, physically are just and are valid two outwardly
opposite statements: in the formula for radius of an orbit the particle mass
should stand in numerator from a point of view of inertia of a particle and in
a denominator from a point of view of maintenance of an angular momentum, if we
shall decrypt it, but it cannot be made, since it is a constant. Unfortunately,
the orthodox physics does not understand physical substance it’s of the
formulas.